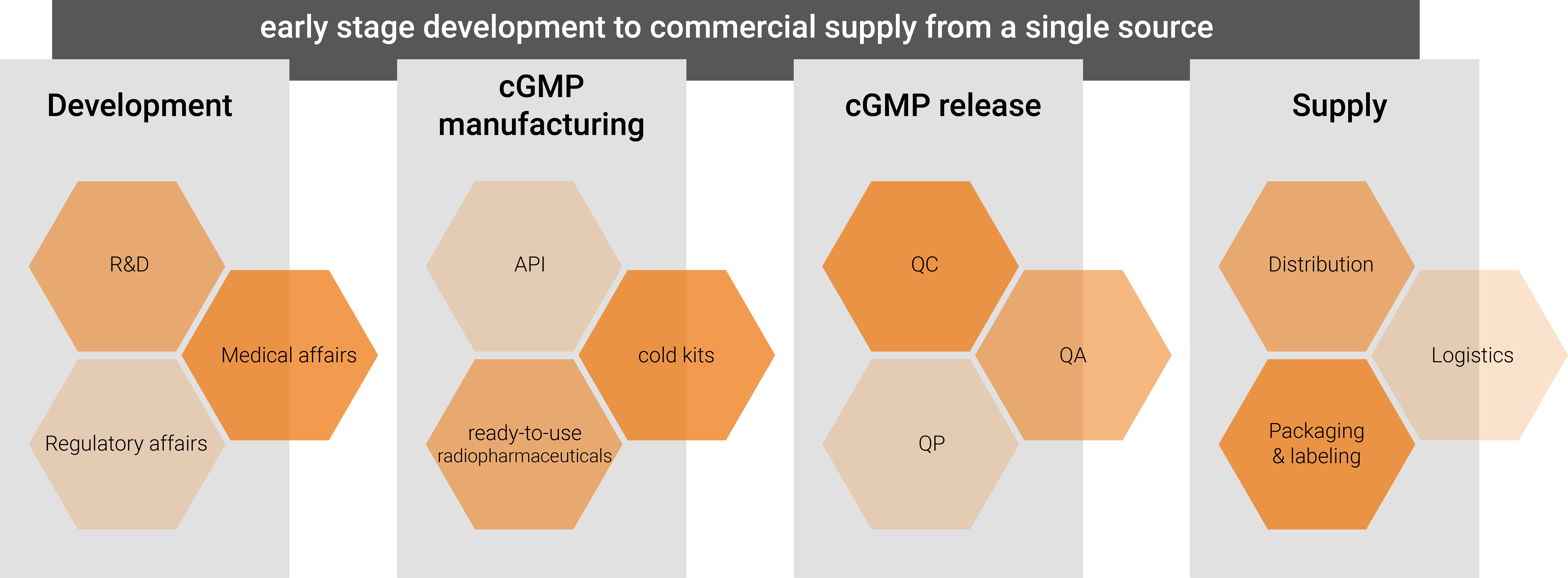
ROTOP has over 20 years’ expertise in the field of product development and product application as well as commercial cGMP production including distribution. At our facility, we combine the complete know-how from pharmaceutical development including regulatory affairs, cGMP manufacturing of APIs and sterile drug products as well as an independent Q-unit with several Qualified Persons (QP) to release drug products.
Based on this widespread experience from earlier stage development to commercial supply, we offer extensive and highly flexible services in the field of CDMO. In this regard, we perform API and lyophilized kit development for PET and SPECT application as well as development and cGMP manufacturing of ready-to-use radiopharmaceuticals. In addition to R&D manufacturing labs for development, we are able to analyze our cold kits and ready-to-use radiopharmaceuticals according to cGMP requirements and additionally prepare regulatory documentation. Our facility is fully FDA inspected and we passed numerous NDA procedures in several countries with different authorities e.g., FDA, EMA, South American or Turkish authorities.
handling license for all isotopes in the field of Theranostics






